
Dikla Sharon, M.Sc., M.A., PMP®Drug Development Expert
A seasoned clinical operations expert with over 18 years of industry experience in clinical R&D, effectively bringing rare diseases products to market.

About Me
I'm a clinical operations and development expert with nearly two decades of experience guiding drug products through complex development stages, from first-in-human trials to regulatory approvals and beyond. I've worked across biotech, pharma, and CRO environments and bring a practical, structured mindset to programs that need clarity and strategic oversight.
What sets me apart is my ability to translate complex requirements into actionable plans. Whether you're developing a novel biologic or an AI-driven diagnostic device, I help you avoid costly missteps by aligning clinical and operational activities with regulatory efforts and business needs.
Based in Vienna and working globally, I offer consulting and fractional services to companies who need deep clinical expertise but aren't ready yet for full-time hires. My goal is always the same: help you build something real, robust, and ready for the next step.
Professional Experience
Over 18 years of industry experience in clinical R&D, with a focus on rare diseases, biologics, and gene therapy products.
Selected Career Highlights
Led clinical operations for a biotech company focused on dermatology and oncology (rare disease) indications.
Key Achievements:
- Spearheaded the creation of comprehensive CDPs for lead candidate products
- Set up and planned all ClinOps-related logistics (e.g., SOPs, finance)
- Vendor selection (key CRO, supporting functions e.g., biostats)
- Liaised with and provided support to early development, CMC, and pre-clinical teams
- Close work with AdBoards and KOLs
Led the Hematology clinical operations team for a major pharmaceutical company focused on rare diseases.
Key Achievements:
- Established the Hematology ClinOps team of 10 CPMs across Vienna and Boston
- Executed and oversaw the entire Hematology ClinOps therapeutic area portfolio
- Set up and conducted gene therapy programs – both FiH and long-term follow up
- Developed ClinOps oversight approach for all CROs
- Supported Shire-Baxalta integration related activities
Managed clinical projects for a pharmaceutical/biotech company focused on Hemophilia.
Key Achievements:
- Lead CPM for the company's top priority pivotal phase III study for marketing approval
- Beat industry standard benchmarks for start-up (~30% faster) and close out (~50% faster)
- Represented ClinOps in the integration process of a newly acquired company
- Piloted aspects of a strategic sponsor-CRO partnership (IQVIA)
Managed clinical trials and regulatory submissions for a CRO, main indications were pain, stroke, and metabolic diseases.
Key Achievements:
- Reviewed and collated all Regulatory and Ethics submissions for Europe, South Africa, and Israel
- Lead CPM for the pivotal study of the first ever FDA-approved drug produced in a plant cell-based expression system (Gaucher)
My Services
Strategic expertise to guide companies through complex clinical development, validation, and regulatory challenges.
For AI Diagnostics & Digital Health: I provide a strategic, end-to-end plan to meet regulatory and validation demands - without overcommitting resources or compromising speed. This leads to efficient execution, confident regulatory engagement, and a clear path to market.
For Early Stage Biotechs: I provide strategic and operational expertise to de-risk and reduce spending, set up clinical operations for success, and ensure internal alignment with business objectives. The result is smarter contracts, better control, and sustained operational performance.
AI Diagnostics & Digital Health
AI-driven companies developing diagnostics or monitoring solutions often face complex and evolving regulatory expectations - especially when their tools fall under classifications that require precision validation in real world setting and, at times, clinical outcome studies.
Validation & Development Planning
Tactical roadmap aligning product development with validation, regulatory, and operational goals. Define evidence generation strategy across analytical, technical, and clinical levels. Identify risks, set realistic timelines, and design study scenarios aligned with budget and commercial priorities.
Study Design Strategy
Design analytical and clinical validation studies that are feasible, fundable, and meet regulatory and operational expectations. Review existing protocols or plans to ensure stakeholder relevance, and real-world applicability.
Product-Workflow Fit Assessment
Identify gaps between how your tool functions and how it would be used in real-world clinical workflows (sites, patients, staff).
CRO/Vendor Selection & RFP Support
Define scope. Identify best vendors. Run RFPs. Evaluate proposals. Negotiate terms and contracts. Set up communication pathways and define deliverables.
Global Clinical Trial Management
From study set up through database lock. Oversight of all activities and management of CROs and vendors.
Project Planning & Milestone Structuring
Interim leadership. Build an execution plan that aligns product, validation, and business goals. Establish oversight structure and performance metrics.
Insights and Resources
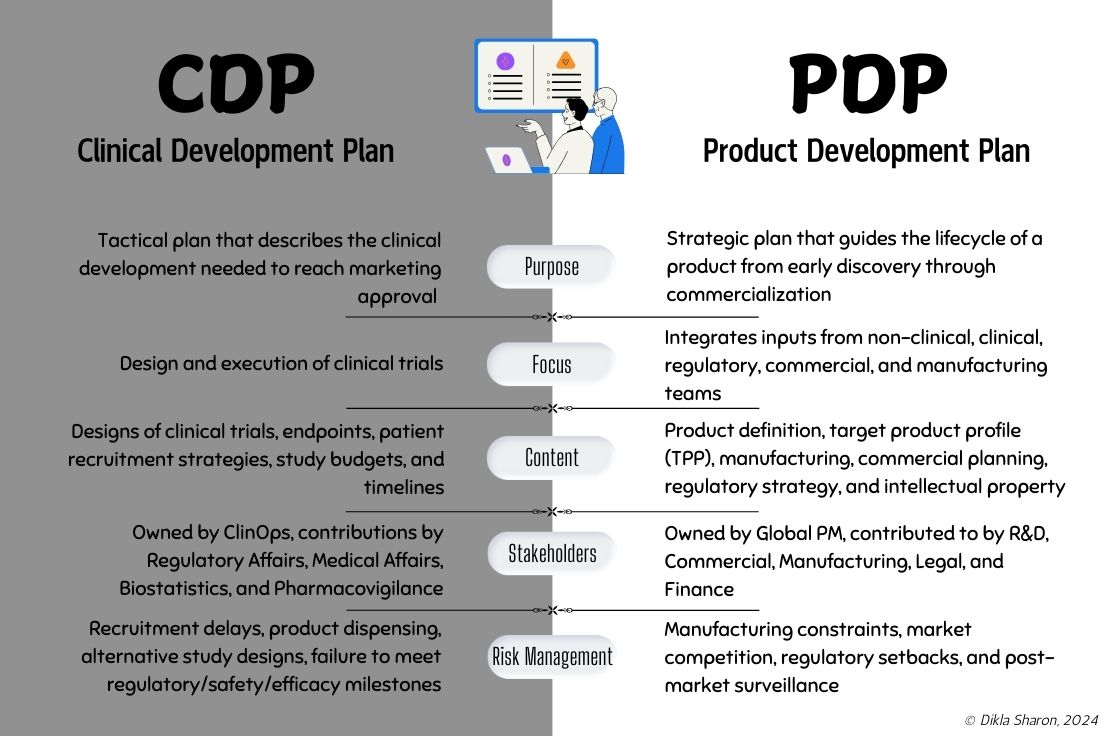
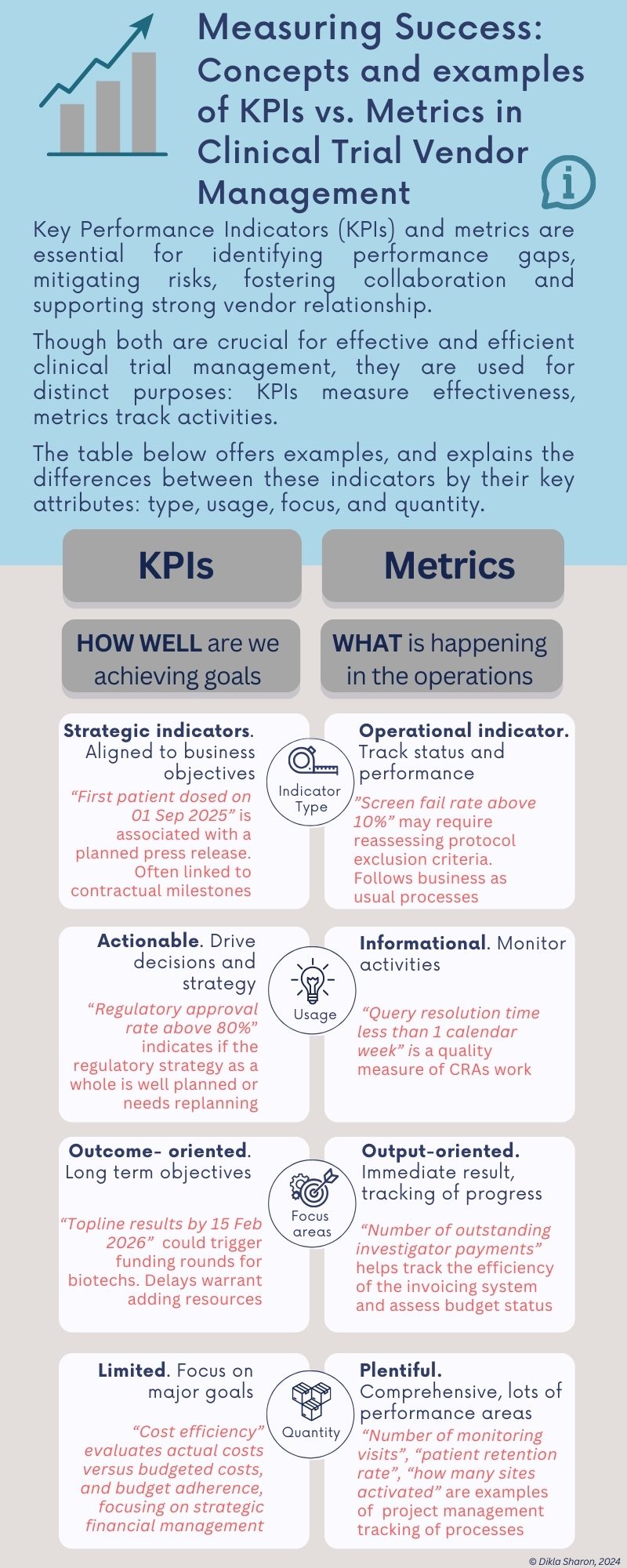
Practical guides and infographics to help you navigate the complexities of clinical development and vendor management.
Get In Touch
Interested in exploring how we might work together, discuss clinical challenges, or simply exchange ideas? I’d welcome the conversation.
Contact Information
Reach out through any of these channels.
dikla@diklasharon.com
Phone
+43 676 7726103
Location
Vienna, Austria